Withdrawn Post Notification ESA-SRB-ANZOS 2025 in conjunction with ENSA
Insulin pump induced lipoatrophy proposed diagnostic and management algorithm (#150)
Lipoatrophy, the localised loss of subcutaneous fat, is a rare complication of insulin therapy, affecting approximately 1% of patients with type 1 diabetes mellitus (T1DM)1. We present a 58-year-old woman with T1DM who developed marked abdominal lipoatrophy four months after commencing insulin pump therapy (see Figure 1). Ultrasound confirmed the affected areas (see Figure 2), and dermatological assessment with incisional biopsy revealed lipoatrophy without inflammation. Management involved changing from a steel to a Teflon cannula, rotating infusion sites, and switching insulin analogues. These interventions led to stabilisation and complete resolution over 16 months.
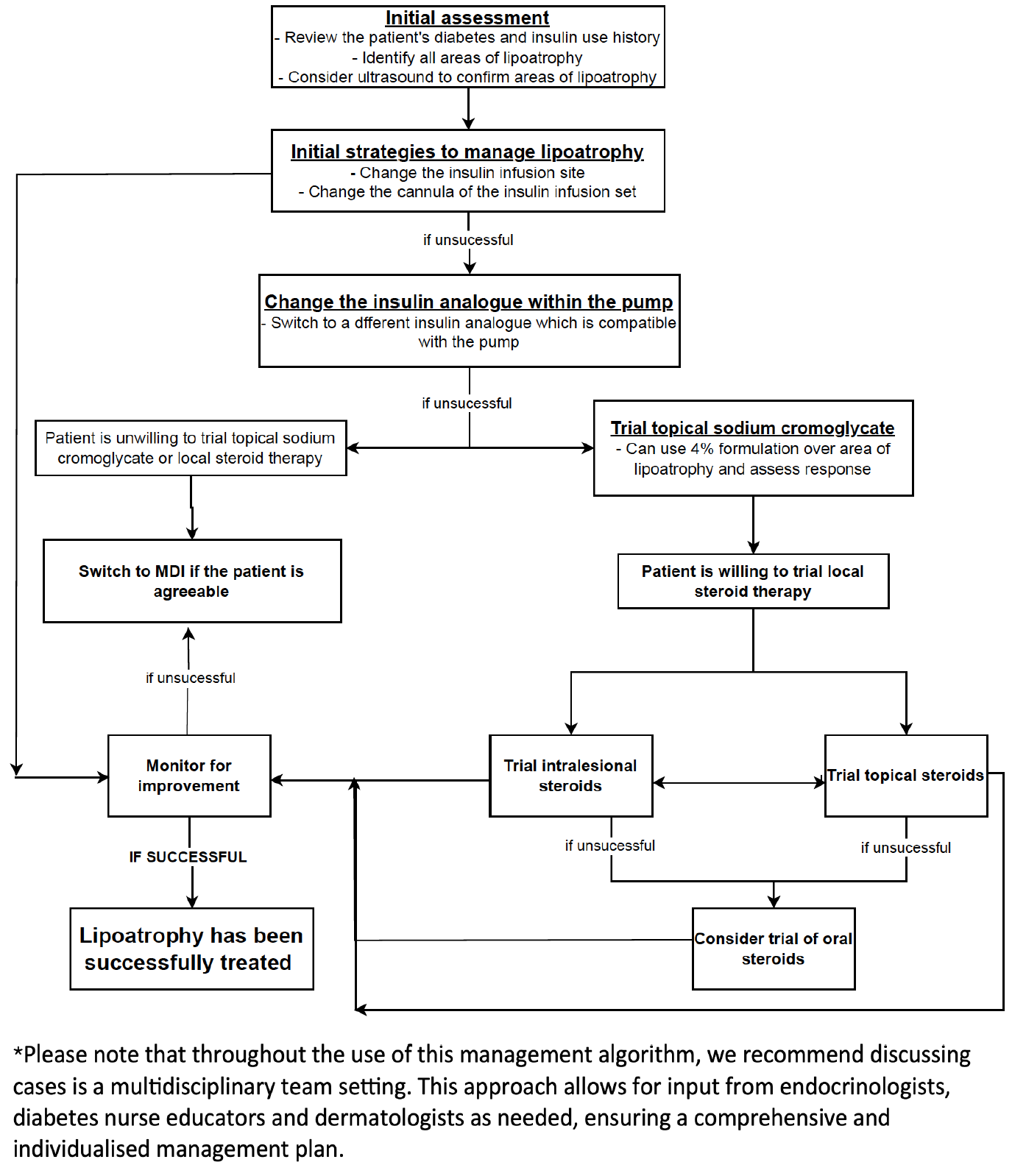
A literature review identified 16 publications on insulin-related lipoatrophy1-16. Based on this and our experience, we propose a diagnostic and management algorithm (see Figure 3). Given the condition’s rarity, multidisciplinary input from endocrinologists, diabetes nurse educators, and dermatologists is recommended.
Initial strategies should include rotating infusion sites every 2–3 days1,4. If lipoatrophy develops, trial changing the cannula type (e.g., steel to Teflon) and insulin analogue. Several reports describe improvement or stabilisation after modifying insulin type in both continuous subcutaneous insulin infusion (CSII)1,2,3 and multiple daily injection (MDI) regimens10, 12, 13.
Topical sodium cromoglycate (SCG) cream has shown benefit, particularly when combined with insulin changes1,6,13. In one case series, 7 of 10 patients improved within 3–24 months, and 4 of 4 insulin-change non-responders showed benefit with SCG.
Corticosteroids—topical, intralesional, or oral—have also been trialled with mixed outcomes5,7,14,15. Due to a better lower side effect profile, topical or intralesional corticosteroids are preferred before considering systemic therapy.
If lipoatrophy persists despite these interventions, switching from insulin pump therapy to MDI should be considered, guided by shared decision-making in a multidisciplinary setting.
Figure 1
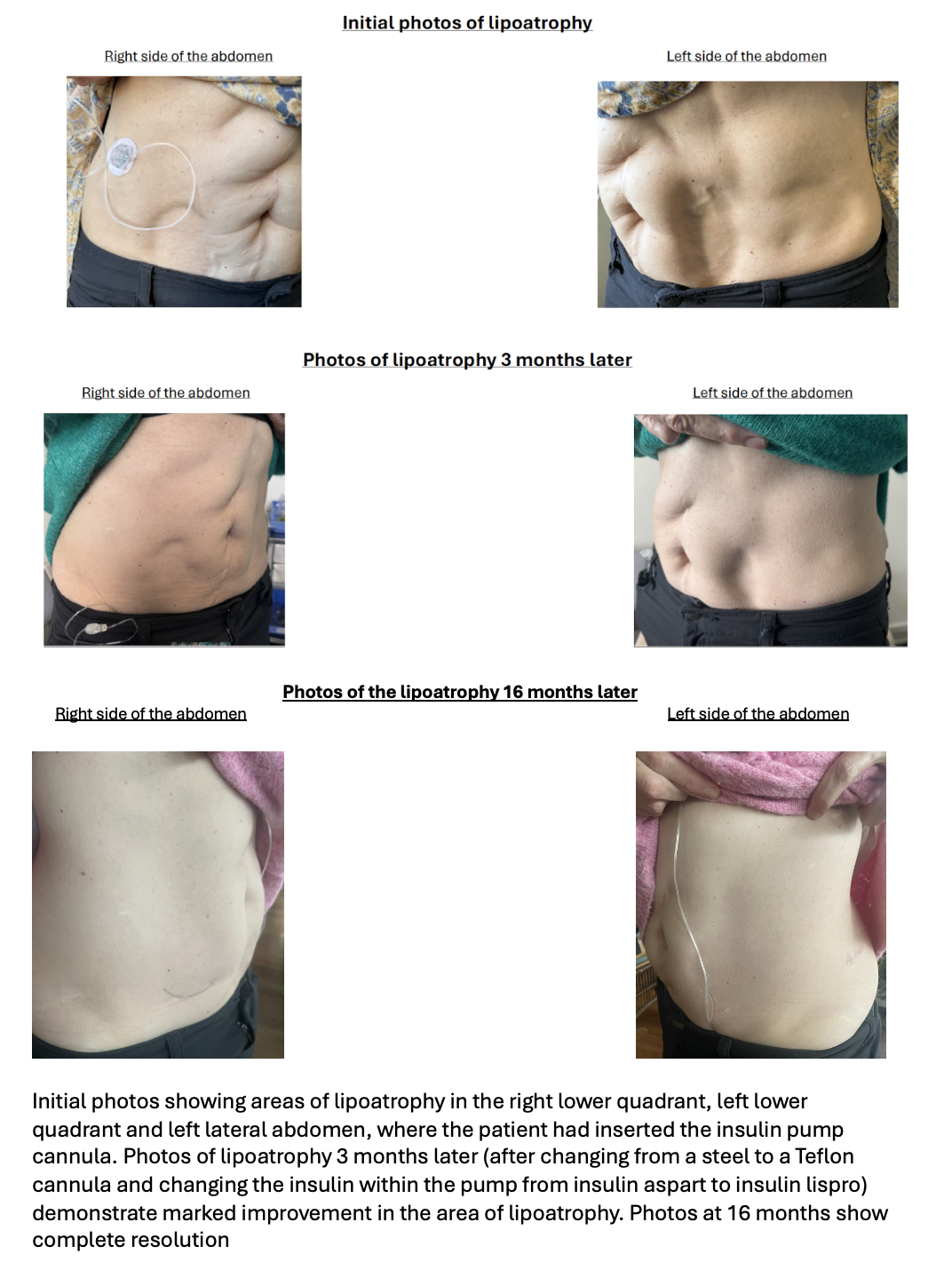
Figure 2
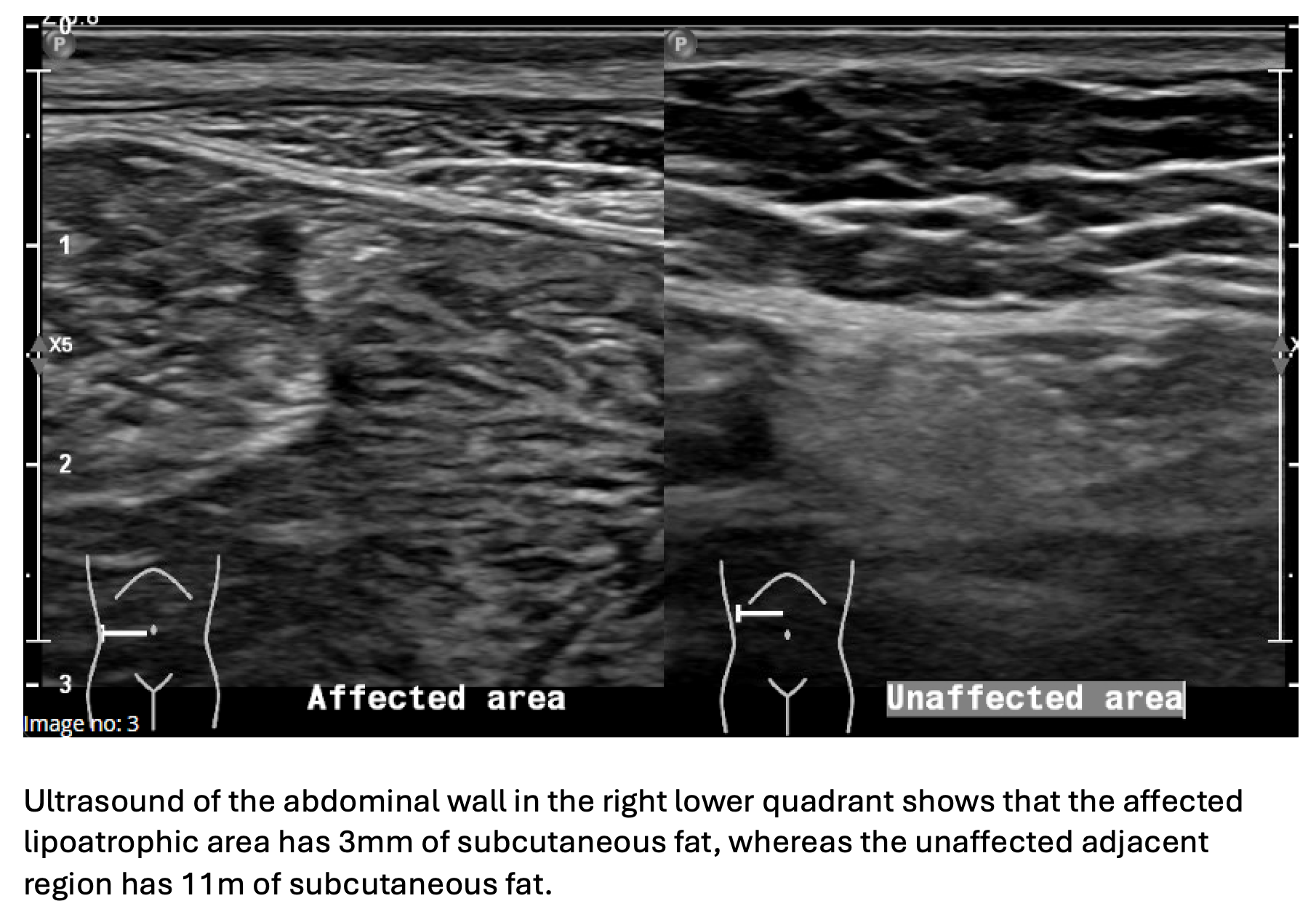
Figure 3
- 1. Xatzipsalti M, Alvertis H, Kourousi G, et al. Lipoatrophy, a rare complication of diabetes: a single-center experience. Hormones (Athens). 2021;20(1):61-69. doi:10.1007/s42000-021-00324-z.
- 2. Griffin ME, Feder A, Tamborlane WV. Lipoatrophy associated with lispro insulin in insulin pump therapy: an old complication, a new cause? Diabetes Care. 2001;24(2):411-412.
- 3. Ampudia-Blasco FJ, Hasbum B, Carmena R. A new case of lipoatrophy with lispro insulin in insulin pump therapy: Is there any insulin preparation free of complications? Diabetes Care. 2003;26(3):953-954. doi:10.2337/diacare.26.3.953.
- 4. Boland X, Chenoweth H, Sulkin T, et al. Progressive and disabling lipoatrophy associated with insulin aspart via a continuous subcutaneous insulin infusion. Pract Diabetes. 2015;32(9):336-337a. doi:10.1002/pdi.1985.
- 5. Chantelau EA, Praetor R, Praetor J, et al. Relapsing insulin-induced lipoatrophy, cured by prolonged low-dose oral prednisone: a case report. Diabetol Metab Syndr. 2011;3(1):33. doi:10.1186/1758-5996-3-33. Accessed from: https://pmc.ncbi.nlm.nih.gov/articles/PMC3251526/pdf/1758-5996-3-33.pdf.
- 6. Lopez X, Castells M, Ricker A, et al. Human insulin analog–induced lipoatrophy. Diabetes Care. 2008;31:442-444. doi:10.2337/dc07-1826.
- 7. Chantelau EA, Prätor R, Prätor J. Insulin-induced localized lipoatrophy preceded by shingles (herpes zoster): a case report. J Med Case Rep. 2014;8:223. doi:10.1186/1752-1947-8-223.
- 8. Milan G, Massarotto A, Astiarraga B, et al. Insulin-induced lipoatrophy and lipoatrophic diabetes: ultrastructural analysis and gene expression profiling of subcutaneous adipose tissue. J Clin Endocrinol Metab. 2010;95(7):E259-E264. doi:10.1210/jc.2009-2301.
- 9. Reeves WG, Allen BR, Tattersall RB. Insulin-induced lipoatrophy: an immune pathogenesis. BMJ. 1980;281(6241):1500-1503.
- 10. Babiker A, Datta V. Lipoatrophy with insulin analogs in type I diabetes. Arch Dis Child. 2011;96(1):101-102. Accessed from: https://adc.bmj.com/content/96/1/101.
- 11. Radermecker RP, Piérard GE, Scheen AJ. Lipodystrophy reactions to insulin: effects of continuous insulin infusion and new insulin analogs. Am J Clin Dermatol. 2007;8(1):21-28. doi:10.2165/00128071-200708010-00003.
- 12. Arranz A, Andia V, López-Guzmán A. A case of lipoatrophy with lispro insulin without insulin pump therapy. Diabetes Care. 2004;27(2):625-626. doi:10.2337/diacare.27.2.625.
- 13. Phua EJ, Lopez X, Ramus J, et al. Cromolyn sodium for insulin-induced lipoatrophy: old drug, new use. Diabetes Care. 2013;36(12):e205. doi:10.2337/dc13-1123. Accessed from: https://diabetesjournals.org/care/article/36/12/e204/33260/Cromolyn-Sodium-for-Insulin-Induced-Lipoatrophy.
- 14. Ramos AJS, Farias MA. Human insulin–induced lipoatrophy: a successful treatment with glucocorticoid. Diabetes Care. 2006;29(4):926-927. doi:10.2337/diacare.29.04.06.dc06-0004.
- 15. Kumar D, Garg SK, Bansal M, et al. Use of dexamethasone in treatment of insulin lipoatrophy. Diabetes. 1977;26(4):296-299. doi:10.2337/diabetes.26.4.296.
- 16. Mu L, Goldman JM. Human recombinant DNA insulin-induced lipoatrophy in a patient with type 2 diabetes mellitus. Case Rep Endocrinol. 2000;6(2):151-152.