Poster Presentation ESA-SRB-ANZOS 2025 in conjunction with ENSA
Effective Use of Pasireotide in Non-Insulinoma Pancreatogenous Hypoglycaemia Syndrome (#153)
A 25-year-old woman was referred for investigation of symptomatic hypoglycaemia. She described episodes of tremor, confusion, and dizziness, occasionally progressing to seizures, which resolved with oral carbohydrates.
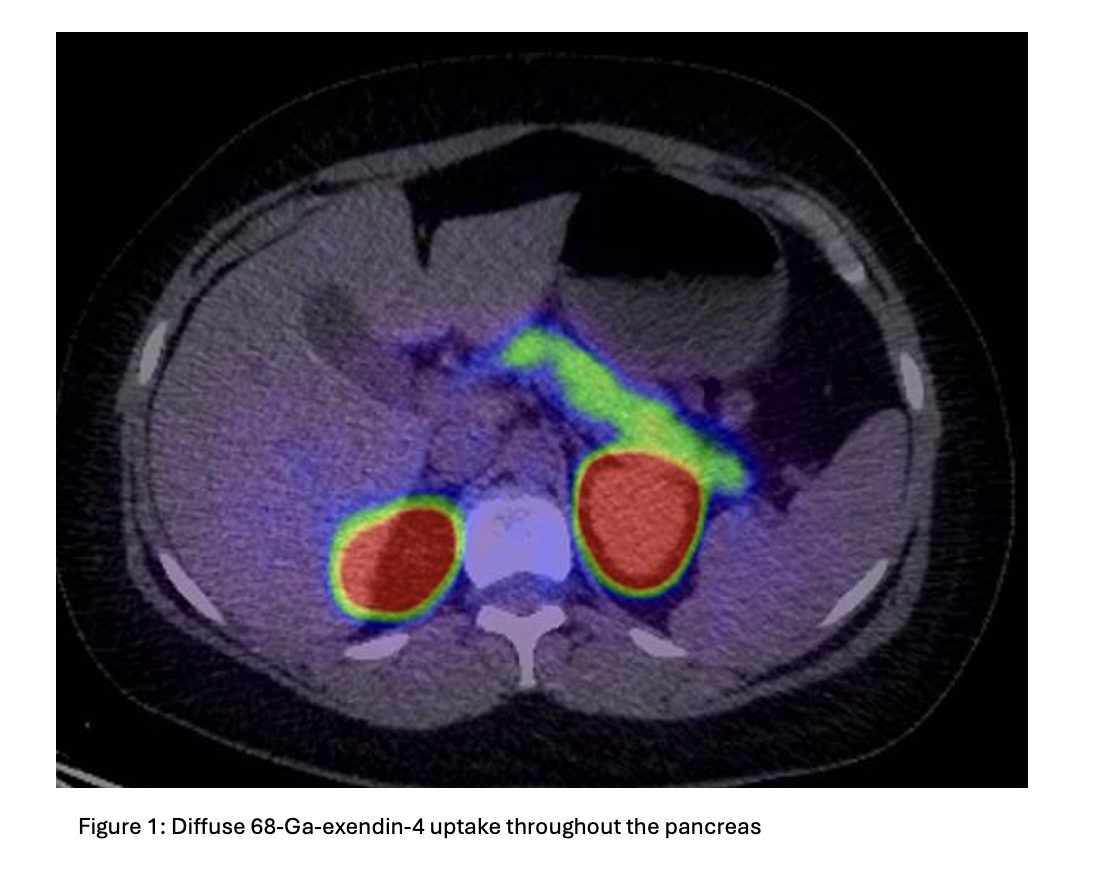
A mixed meal test was non-diagnostic, but a supervised 72-hour fast confirmed hyperinsulinaemic hypoglycaemia (table 1). Endoscopic ultrasound and CT abdomen/pelvis were unremarkable. Ga-68 GLP-1 receptor PET/CT demonstrated diffuse uptake throughout the pancreas without discrete mass, consistent with nesidioblastosis (figure 1). Genetic testing did not reveal a monogenic cause.
Medical therapy with diazoxide was ineffective. A calcium channel blocker was ceased due to postural hypotension and lack of efficacy. Octreotide produced transient benefit only. Surgical resection was not pursued due to high operative risk and uncertain benefit.
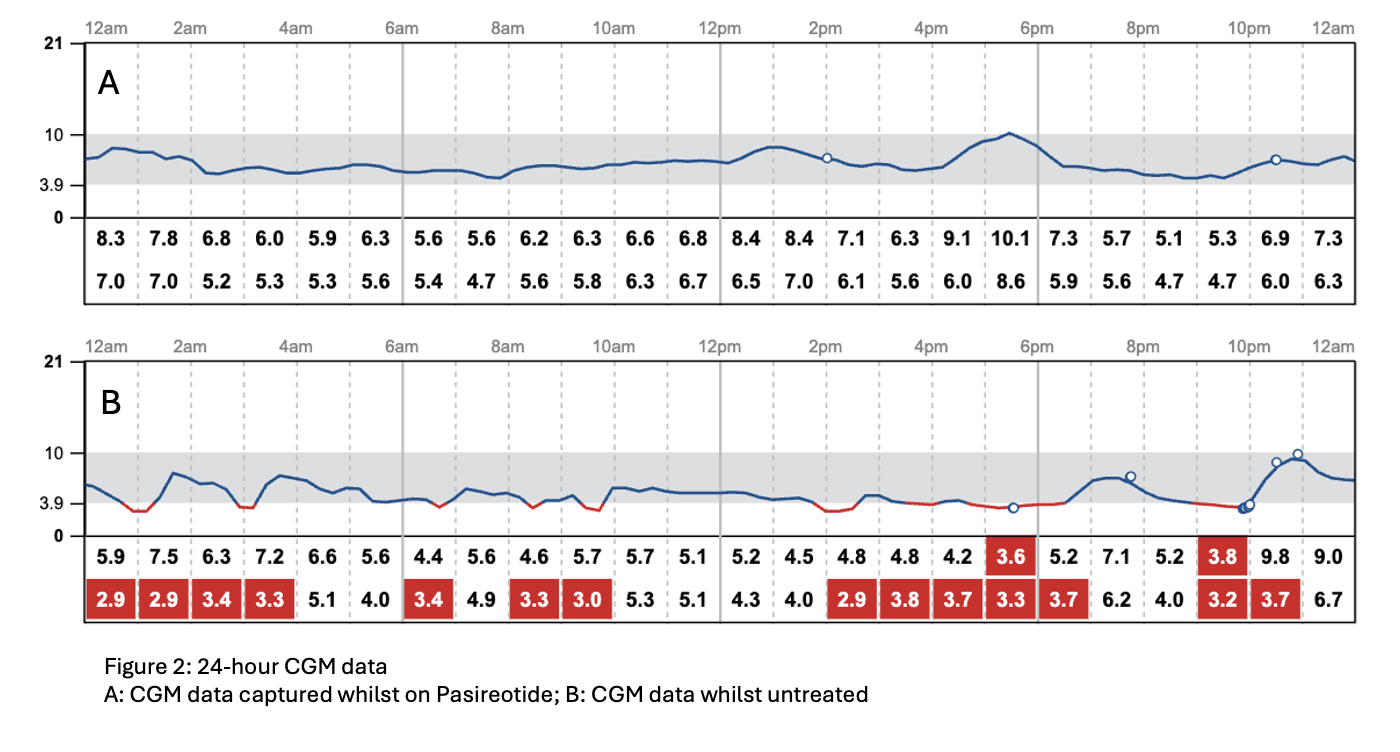
In 2024, Pasireotide—a second-generation somatostatin analogue—was commenced, resulting in complete resolution of hypoglycaemia and restoration of quality of life. Symptom recurrence during a treatment break supported its clinical efficacy (figure 2).
Discussion:
Non-insulinoma pancreatogenous hypoglycaemia syndrome (NIPHS) is a rare cause of hyperinsulinaemic hypoglycaemia in adults and can be congenital, idiopathic or occur post gastric bypass. Surgical resection may be ineffective and carries substantial risk. Dietary interventions and medical therapies such as diazoxide, calcium channel blockers, and glucocorticoids are variably effective and frequently limited by adverse effects.
Somatostatin exerts its effects via five receptor subtypes. SSTR2 and SSTR5 are particularly relevant to glucose homeostasis: both inhibit insulin secretion from pancreatic beta cells, while SSTR2 also suppresses glucagon activity. First-generation analogues (e.g. Octreotide) predominantly bind SSTR2, reducing insulin but potentially worsening hypoglycaemia via glucagon suppression.
Pasireotide has markedly higher affinity for SSTR5 and relatively lower affinity for SSTR2, thereby more selectively inhibiting insulin secretion. Although hyperglycaemia is a common side effect, this pharmacologic profile offers a therapeutic advantage in NIPHS. Our case illustrates successful use of Pasireotide in refractory NIPHS, supporting its role in select cases.
- Dieterle, M. P., et al. (2023). "Diffuse, Adult-Onset Nesidioblastosis/Non-Insulinoma Pancreatogenous Hypoglycemia Syndrome (NIPHS): Review of the Literature of a Rare Cause of Hyperinsulinemic Hypoglycemia." Biomedicines 11(6).
- Gomes-Porras, M., et al. (2020). "Somatostatin Analogs in Clinical Practice: a Review." Int J Mol Sci 21(5).
- Hendren, N. S., et al. (2018). "Pasireotide for the treatment of refractory hypoglycaemia from malignant insulinoma." Clinical Endocrinology 88(2): 341-343.
- Hofland, J., et al. (2023). "European Neuroendocrine Tumor Society 2023 guidance paper for functioning pancreatic neuroendocrine tumour syndromes." Journal of Neuroendocrinology 35(8): e13318.
- Hofland, J., et al. (2023). "Approach to the Patient: Insulinoma." The Journal of Clinical Endocrinology & Metabolism 109(4): 1109-1118.
- Howarth, S., et al. (2025). "Managing Hypoglycaemia in Patients With Insulinoma—A Tertiary Centre Experience and Review of the Literature." Clinical Endocrinology 102(3): 344-354.
- Lesche, S., et al. (2009). "Differential Effects of Octreotide and Pasireotide on Somatostatin Receptor Internalization and Trafficking in Vitro." The Journal of Clinical Endocrinology & Metabolism 94(2): 654-661.
- Oziel-Taieb, S., et al. (2022). "Pasireotide for Refractory Hypoglycemia in Malignant Insulinoma- Case Report and Review of the Literature." Frontiers in Endocrinology Volume 13 - 2022.
- Vega-Beyhart, A., et al. (2025). "Efficacy and Safety of Pasireotide in Insulinoma-Associated Hypoglycemia." The Journal of Clinical Endocrinology & Metabolism 110(7): e2109-e2120.
- Zhang, B., et al. (2024). "Structure and Function of Somatostatin and Its Receptors in Endocrinology." Endocrine Reviews 46(1): 26-42.