Poster Presentation ESA-SRB-ANZOS 2025 in conjunction with ENSA
Osilodrostat in ectopic ACTH syndrome: a case series using a block and replace strategy and literature review (#154)
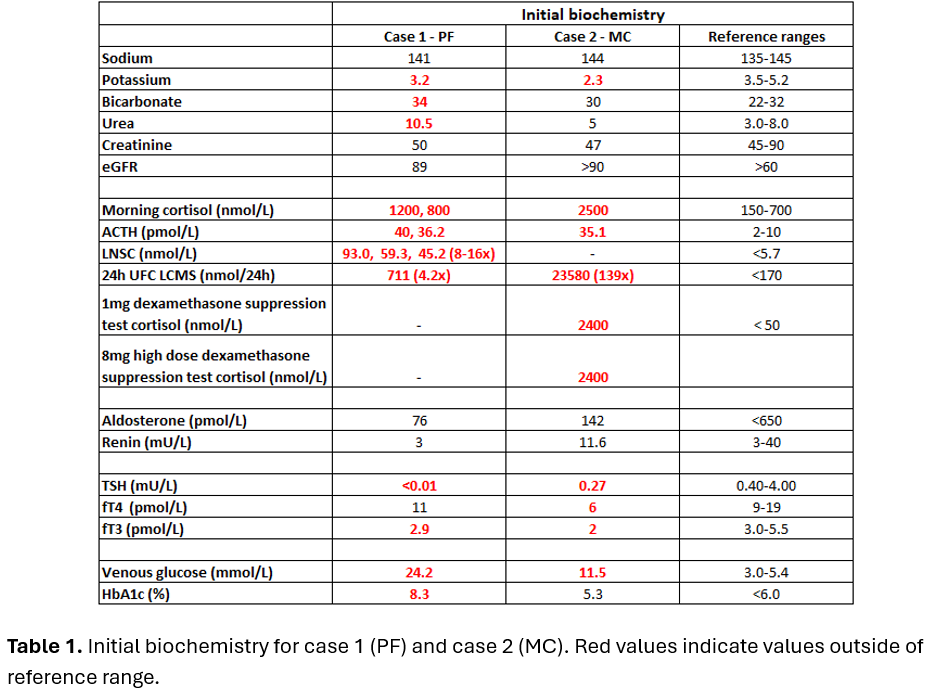
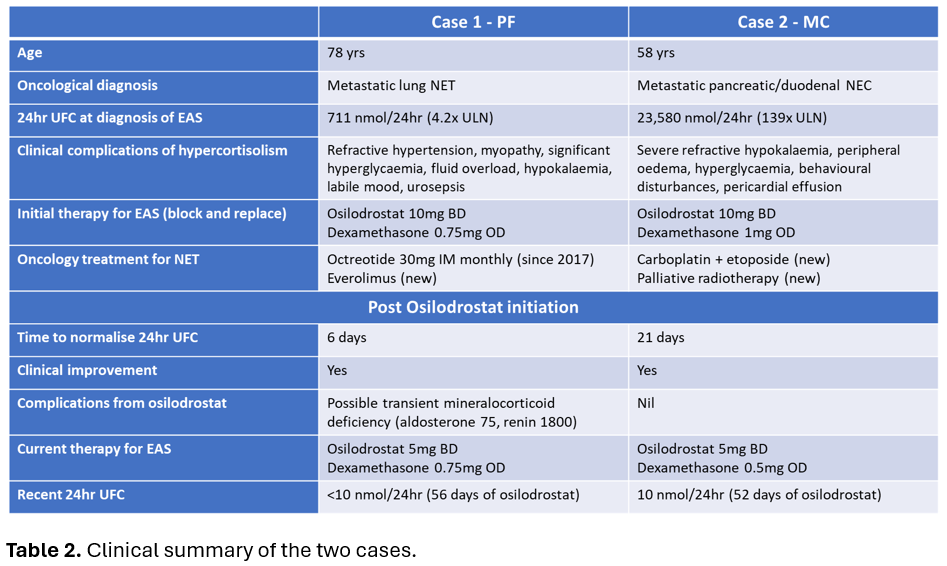
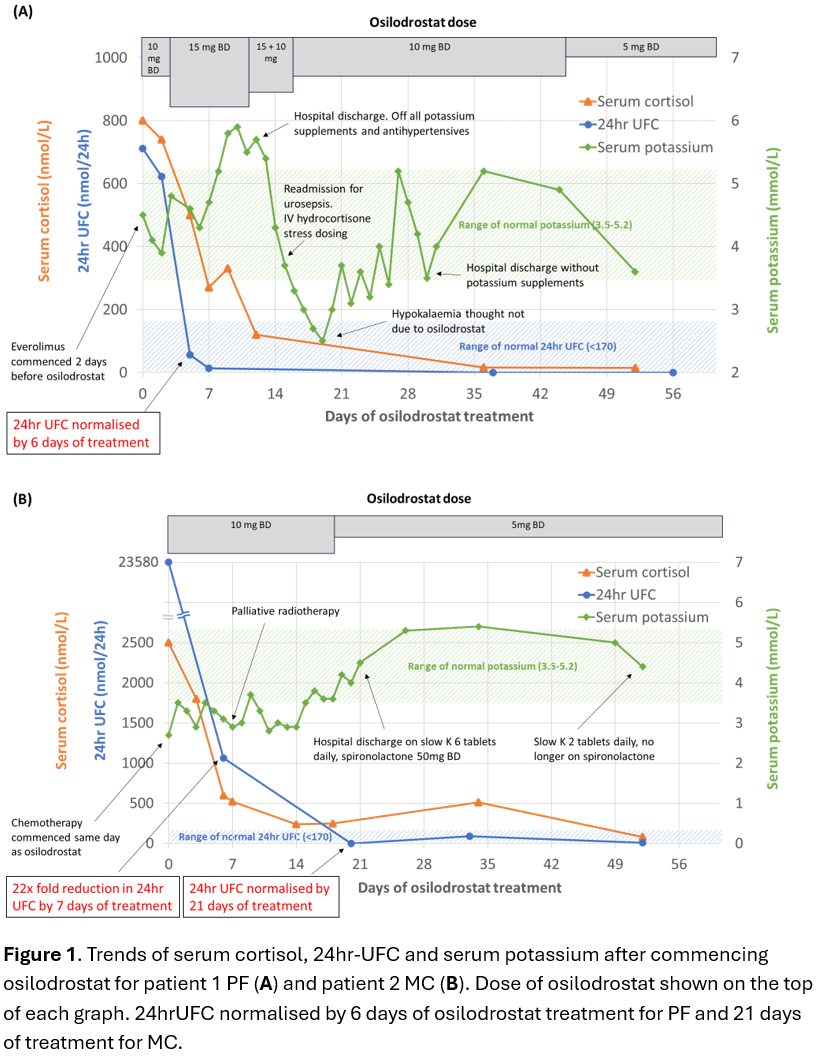
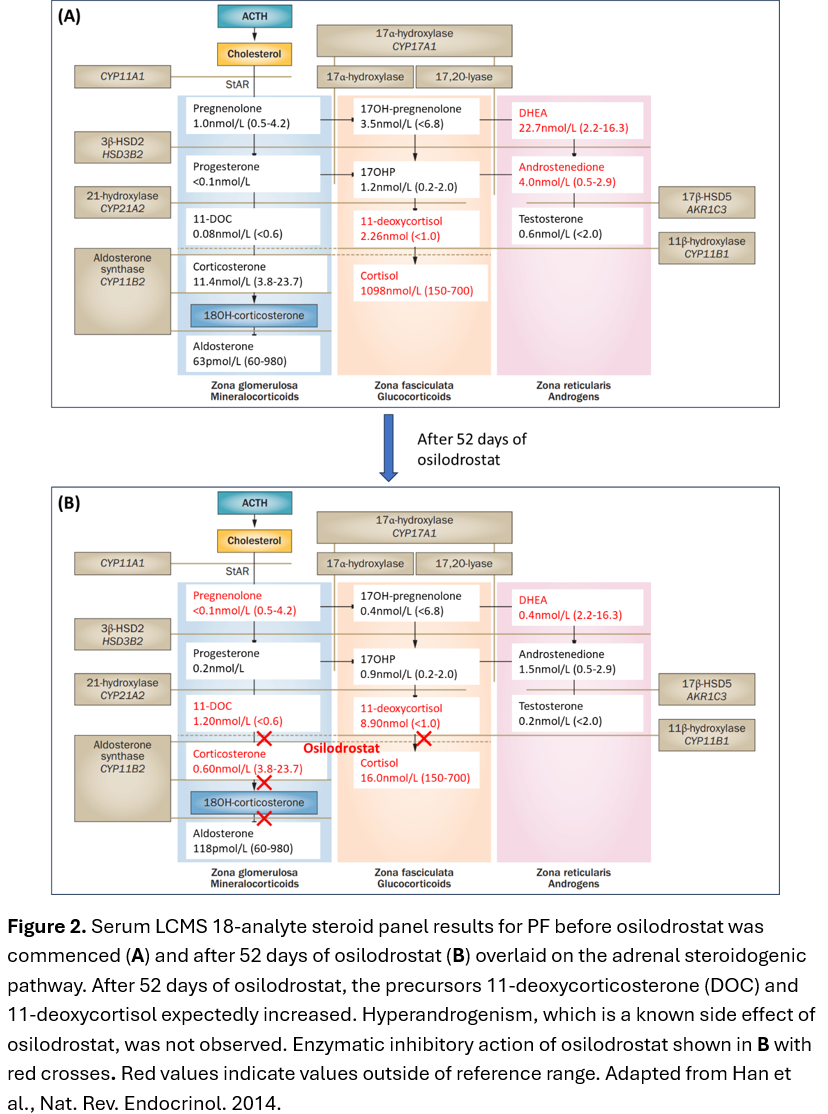
Case 1: PF, a 78-year-old female was admitted with treatment-resistant hypertension on a background of metastatic lung neuroendocrine tumour (NET). Ectopic ACTH syndrome (EAS) was diagnosed in the setting of raised late-night salivary cortisol (LNSC) 16×ULN, 24-hour urinary free cortisol (24hrUFC) 4.2×ULN, ACTH 4×ULN(Table 1) and rapid onset of complications of hypercortisolism(Table 2). PF was commenced on osilodrostat block and replace strategy with 10mg BD and dexamethasone 0.75mg daily(Table 2). 24hrUFC normalised after 6 days of treatment(Figure 1A). After 52 days of osilodrostat, an expected increase in precursors (11-deoxycorticosterone and 11-deoxycortisol) without hyperandrogenism was observed(Figure 2).
Case 2: MC, a 58-year-old female was admitted for epigastric pain on a background of recently diagnosed metastatic pancreatic/duodenal neuroendocrine carcinoma. Due to refractory hypokalaemia, EAS was suspected and confirmed on biochemistry including 24hrUFC 139×ULN(Table 1). Osilodrostat block and replace strategy with 10mg bd and dexamethasone 1mg daily was commenced(Table 2) and 24hrUFC normalised after 21 days(Figure 1B).
Discussion: We described two cases of EAS successfully treated with osilodrostat block and replace strategy without major adverse effects.
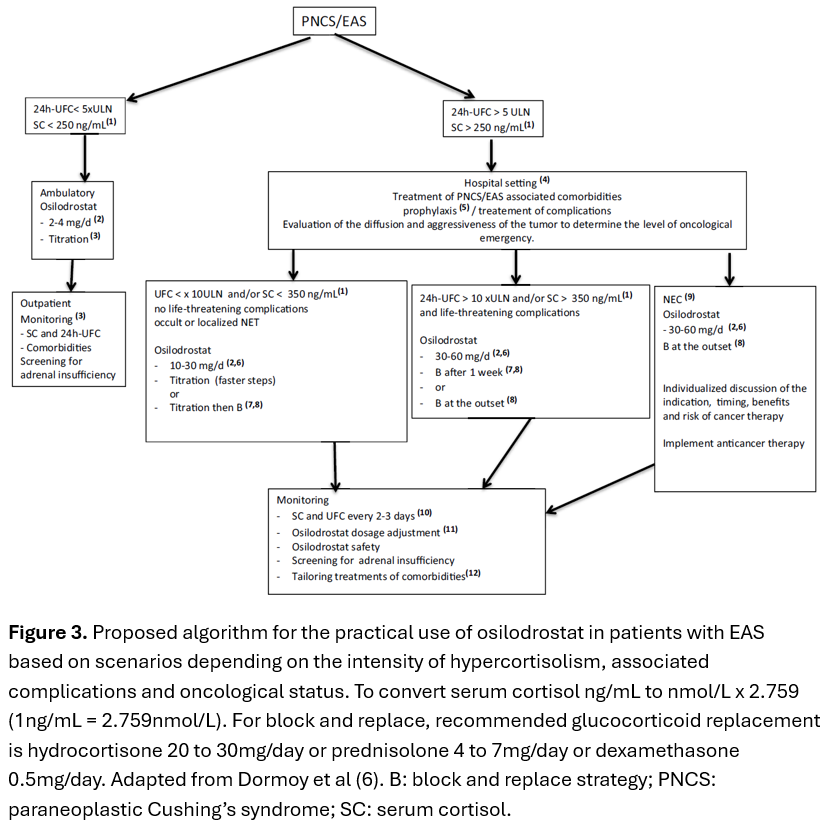
EAS is associated with rapid onset of life-threatening complications from severe hypercortisolism. Definitive management is NET resection, however, NETs are often metastatic or occult/unresectable(1). Steroidogenesis inhibitors, rather than bilateral adrenalectomy, are frequently used in this setting, although some cases are refractory or intolerant of combination therapy of ketoconazole and metyrapone(1). Osilodrostat, a novel, potent oral reversible steroidogenesis inhibitor of 11β-hydroxylase and aldosterone synthase, is effective in treating Cushing’s disease(2, 3), but has limited data in EAS(4, 5) which may require higher doses and faster dose titrations. Dormoy and colleagues demonstrated that in 33 patients with EAS, osilodrostat significantly reduced 24hrUFC and improved clinical features including cases refractory to other steroidogenesis inhibitors, however 24% developed adrenal insufficiency(6). An algorithm to treat EAS with osilodrostat was proposed(Figure 3)(6).
- Fleseriu M, Auchus RJ, Bancos I, Biller BMK. Osilodrostat Treatment for Adrenal and Ectopic Cushing Syndrome: Integration of Clinical Studies With Case Presentations. J Endocr Soc. 2025;9(4):bvaf027.
- Pivonello R, Fleseriu M, Newell-Price J, Bertagna X, Findling J, Shimatsu A, et al. Efficacy and safety of osilodrostat in patients with Cushing's disease (LINC 3): a multicentre phase III study with a double-blind, randomised withdrawal phase. Lancet Diabetes Endocrinol. 2020;8(9):748-61.
- Gadelha M, Bex M, Feelders RA, Heaney AP, Auchus RJ, Gilis-Januszewska A, et al. Randomized Trial of Osilodrostat for the Treatment of Cushing Disease. The Journal of Clinical Endocrinology & Metabolism. 2022;107(7):e2882-e95.
- Haissaguerre M, Puerto M, Nunes ML, Tabarin A. Efficacy and tolerance of osilodrostat in patients with severe Cushing's syndrome due to non-pituitary cancers. Eur J Endocrinol. 2020;183(4):L7-L9.
- Tanaka T, Satoh F, Ujihara M, Midorikawa S, Kaneko T, Takeda T, et al. A multicenter, phase 2 study to evaluate the efficacy and safety of osilodrostat, a new 11β-hydroxylase inhibitor, in Japanese patients with endogenous Cushing's syndrome other than Cushing's disease. Endocr J. 2020;67(8):841-52.
- Dormoy A, Haissaguerre M, Vitellius G, Do Cao C, Geslot A, Drui D, et al. Efficacy and Safety of Osilodrostat in Paraneoplastic Cushing Syndrome: A Real-World Multicenter Study in France. The Journal of Clinical Endocrinology & Metabolism. 2022;108(6):1475-87.