Poster Presentation ESA-SRB-ANZOS 2025 in conjunction with ENSA
Local community and social media advertisement may be an efficient and cost-effective strategy to recruit postmenopausal women into clinical trials: insights from the ROLEX-DUO study (#136)
Recruiting a sufficient sample size is a major barrier in conducting investigator-initiated clinical trials (1). Despite this, there is scarce literature on success and costs of various recruitment strategies, including use of social media in recruiting older community cohorts, which can add to challenges in planning and budgeting for clinical trials. Hence, we present a preliminary analysis of recruitment for an ongoing clinical trial in postmenopausal women.
ROLEX-DUO is a placebo-controlled randomised controlled trial (RCT) conducted at RNSH/Westmead Hospitals, Sydney (2, 3). The primary aim is to assess whether high-intensity resistance/impact exercise can enhance effects of romosozumab on bone mineral density in postmenopausal women over 8-months (n=75). Eligible women are 50-80yrs with osteoporosis/osteopenia and able to commit to a twice-weekly local exercise program. Women are excluded if any recent fragility fracture, or current/recent osteoporosis pharmacotherapy. Recruitment data is collected prospectively and efficiency of recruitment strategies based on enrolment conversion rates (% expressions-of-interest (EOIs) converted to participant enrolment).
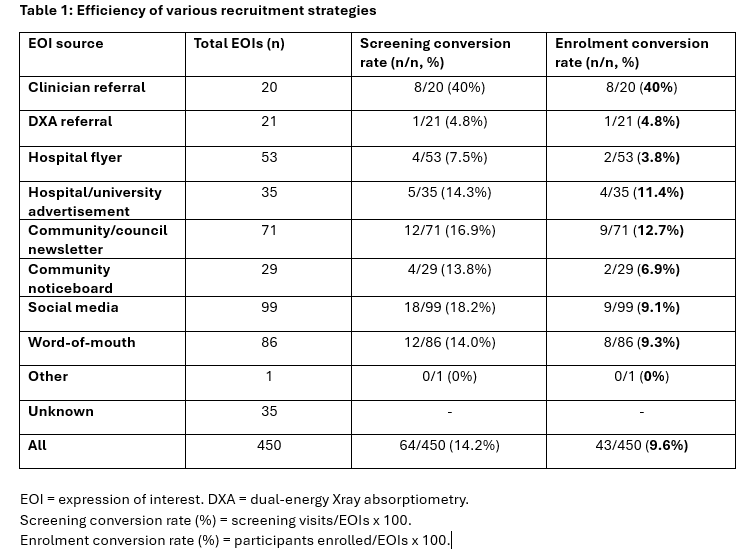
Between March 2024-May 2025, out of 450 EOIs, n=64 women underwent screening assessments of which n=43 participants were enrolled (conversion rate 9.6%) (Table 1). Clinician referrals were the most efficient recruitment source (40%), followed by community/council newsletters (12.7%), hospital/university advertisement (11.4%), word-of-mouth (9.3%) and social media (9.1%). Most frequent reasons for exclusion were other osteoporosis/hormonal pharmacotherapy (n=107), no longer interested (n=89), already participating in resistance exercise (n=48), age out of range/perimenopausal (n=40) or unable to be contacted (n=38). A total $1,070 AUD has been spent on recruitment.
In this preliminary recruitment analysis of an RCT, local community-based and geo-targeted social media advertisement have been efficient strategies in recruiting an older postmenopausal cohort. Recruitment at this scale may be performed with minimal costs. Greater transparency and reporting of recruitment data may better inform future clinical trial research.
- Bessell E, Markovic TP, Caterson ID, Hendy C, Burk J, Picone T, Fuller NR. Cost-effectiveness analysis of recruitment strategies in a large diabetes prevention trial conducted across two sites in Sydney, Australia. Contemp Clin Trials. 2024;137:107421. doi: 10.1016/j.cct.2023.107421.
- Kumar S, Beck BR, Nery L, Byth K, Elhindi J, Wood C, Fuller OK, Clifton-Bligh RJ, Girgis CM. Study protocol for the ROLEX-DUO randomised placebo-controlled trial: ROmosozumab Loaded with EXercise - DUal effects on bone and muscle in postmenopausal Osteoporosis and Osteopenia. BMJ Open. 2024;14(8):e086708. doi: 10.1136/bmjopen-2024-086708.
- Kumar S, Smith C, Clifton-Bligh RJ, Beck BR, Girgis CM. Exercise for Postmenopausal Bone Health - Can We Raise the Bar? Curr Osteoporos Rep. 2025;23(1):20. doi: 10.1007/s11914-025-00912-7.