Oral Presentation ESA-SRB-ANZOS 2025 in conjunction with ENSA
Weight-independent benefits of semaglutide on histology and non-invasive tests in participants with biopsy-defined MASH: Post hoc analysis of the ESSENCE trial part 1 (127990)
Aim: The phase 3 ESSENCE trial (NCT04822181) reported positive interim results in 800 randomised participants with F2/F3 MASH receiving once-weekly semaglutide 2.4 mg vs placebo. In this post hoc analysis, we assessed the weight dependency of the effects of semaglutide 2.4 mg on non-invasive tests (NIT)s & histology after 72 weeks, using weight loss-independent & -dependent pathways as covariates.
Methods: NITs & biopsies were assessed at baseline & week 72. MASH-related NIT responder endpoints were change in ALT( ≥17-unit reduction) & FibroScan-AST (FAST) score (≥0.22 reduction). Fibrosis-related NIT responder endpoints were change in liver stiffness measurement (VCTE -30%) & Enhanced Liver Fibrosis (ELF) score (≥0.5-unit reduction). Histologic endpoints included MASH resolution & improvement in fibrosis. All endpoints were assessed using logistic regression at week 72 with treatment as exposure, % weight loss from baseline to w72 as mediator, baseline T2D status, fibrosis stage, & body weight as covariates. The total & weight loss-independent & -dependent effect sizes were calculated as odds ratios (ORs), & missing data were omitted. Data are based on the full analysis set from the on-treatment observation period.
Results:
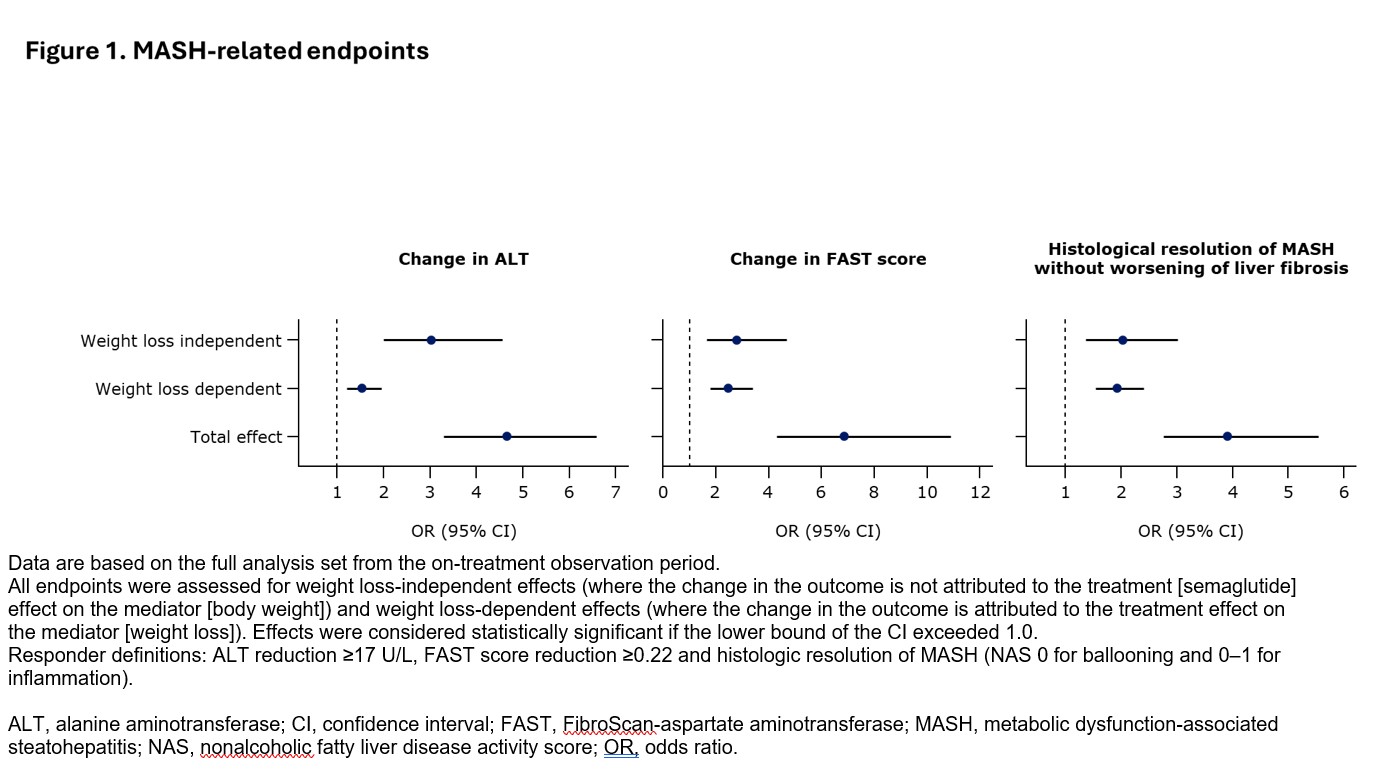
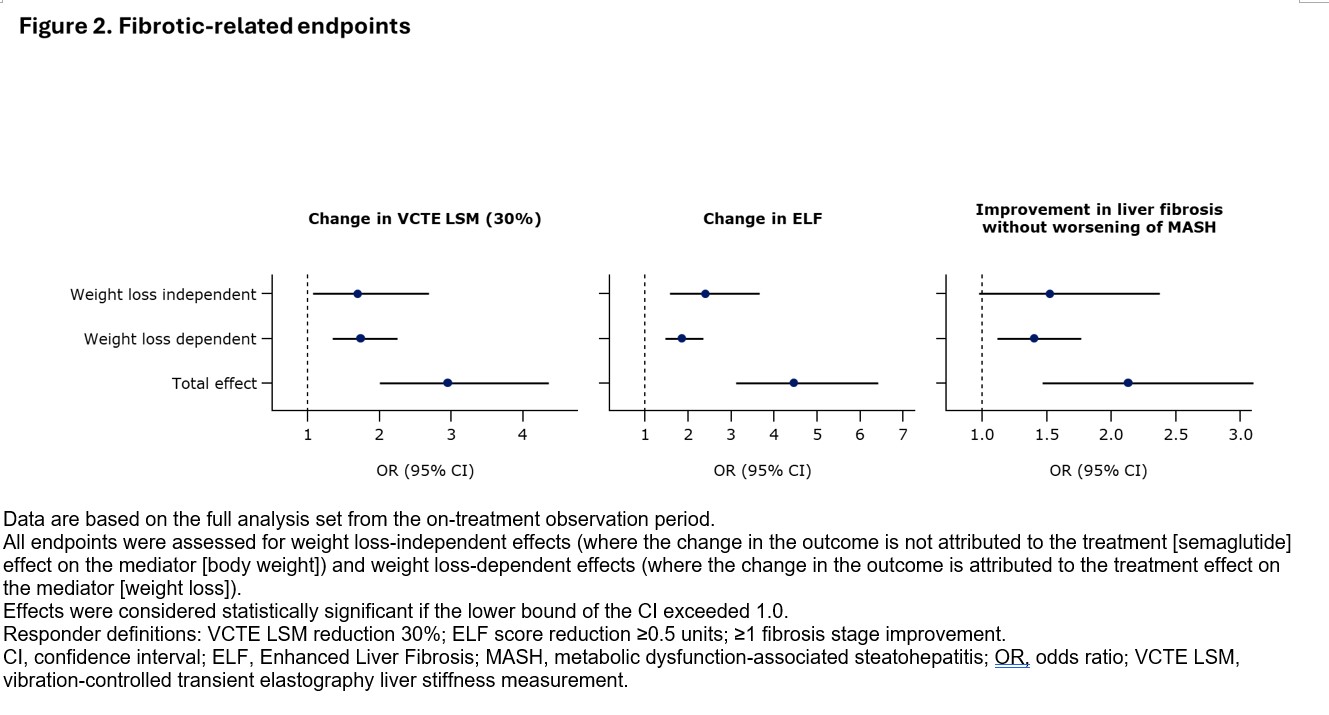
Conclusion: Semaglutide 2.4 mg improved MASH-related histological & NIT endpoints & fibrosis-related NIT endpoints through equal contributions of weight loss-independent & -dependent metabolic mechanisms, with effects beyond weight loss.